Fig. 3
Download original image
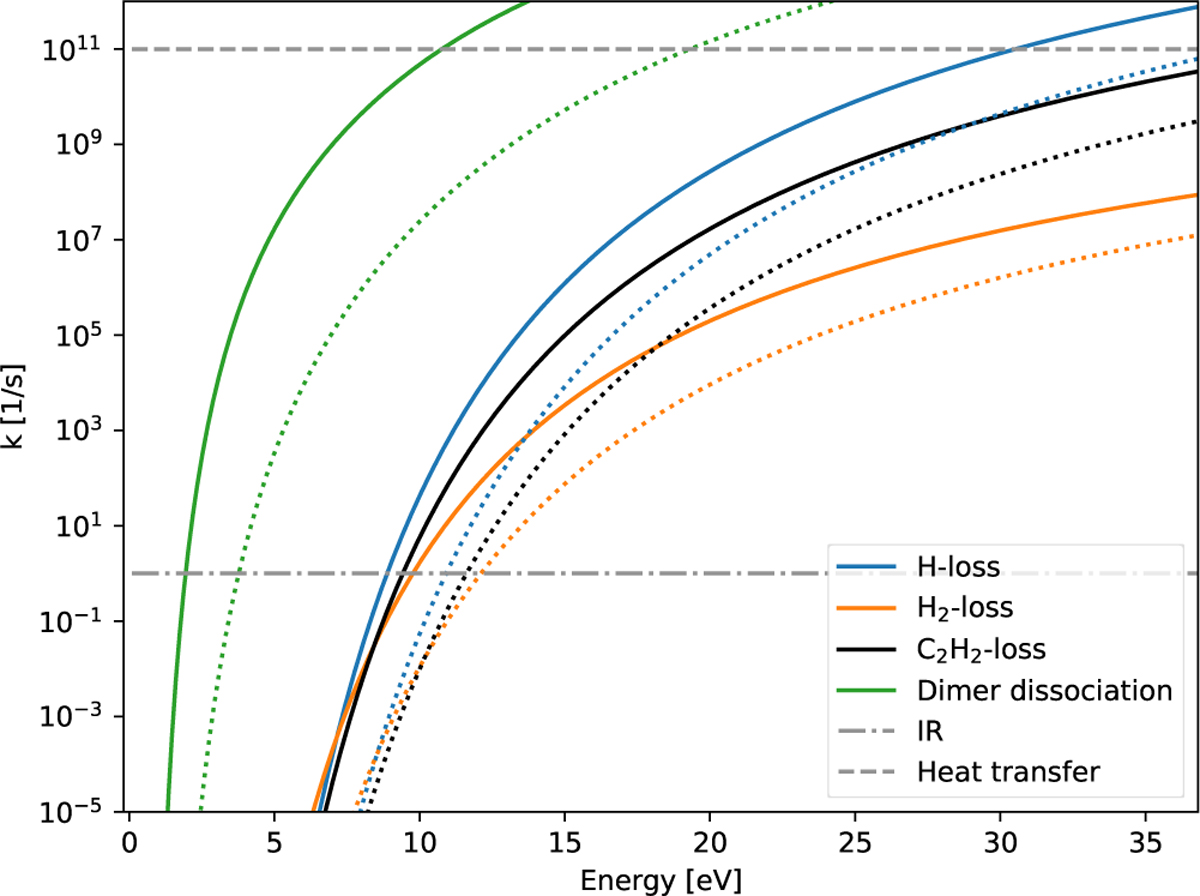
Comparison of dissociation rates for coronene C24H12 (solid lines) and ovalene C36H18 (dashed lines). Only for low energies where H and C dissociation starts to appear do H2 and C2H2 loss have comparable rates to H loss; for high energies H loss always dominates. Dissociation of a dimer is always favoured because of the much weaker binding energy between two PAHs. For ovalene, the reactions appear at nearly the same temperature but at higher energies because of the larger heat capacity. Hence, the increasing size of PAHs and PAH clusters increases their stability against X-rays as the deposited energy is independent of the structure. The dissociation rate for clusters additionally depends on the size of the cluster. The typical rates for IR cooling and energy transfer to dust grains are indicated by the grey lines.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


