Fig. 8
Download original image
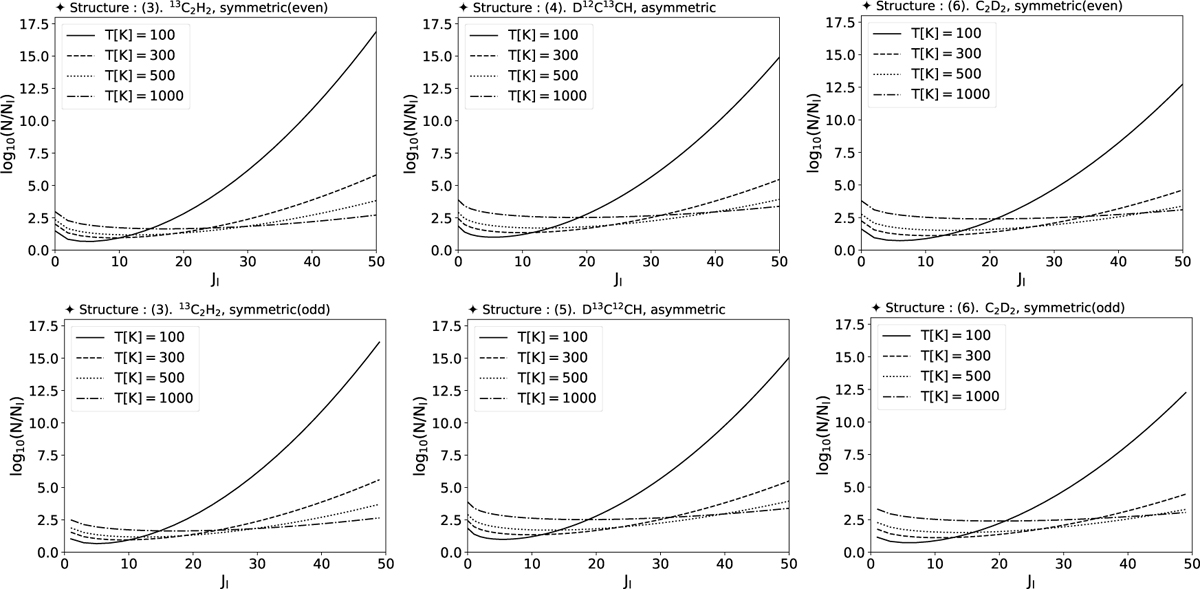
Predicted log10(Nl/gl) values for various isotopologs of acetylene, calculated as a function of the lower rotational quantum number, Jl at four temperatures, T = 100 (solid), 300 (dashed), 500 (dotted), and 1000 (dot-dashed lines) K. The left column corresponds to 13C2H2 (Isotopolog “3”), with separate plots for even (top) and odd (bottom) rotational states. The middle column shows results for D12C13 CH (Isotopolog “4”) and D13C12CH (Isotopolog “5”), both of which are asymmetric molecules. The right column corresponds to C2D2 (Isotopolog “6”), with separate plots for even (top) and odd (bottom) rotational states. The curves are based on partition functions and energy spectra derived from quantum chemical calculations. Even states start at Jl = 0, while odd states start at Jl = 1, reflecting the symmetry properties of the isotopologs. These plots illustrate the expected population distributions across a range of temperatures, providing insights into the ro-vibrational characteristics of these acetylene isotopologs.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


