Table 2
Molecular column densities in IRAS 16293E.
| Method | Molecule | Tex (K) | N (cm−2) |
|---|---|---|---|
| RADEX | CH3OH E | 6.54±0.32 | (6.17±1.95) × 1013 |
| CH3OH A | 6.60±0.47 | (5.94±0.20) × 1013 | |
| 13CH3OH E | 6.75±1.34 | (8.00±1.50) × 1011 | |
| 13CH3OH A | 6.64±1.28 | (8.50±0.15) × 1011 | |
| HCOOCH3 A | 26.0±4.59 | (2.45±0.05) × 1012 | |
| RD | CH3CHO A | 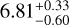 |
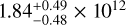 |
| CH2DOH e0 | 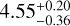 |
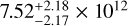 |
|
| LTE | CHD2OH e0 | 4.55 | (2.64±0.83) × 1012 |
| CH3OCH3 EE | 10 | (1.30±0.39) × 1012 | |
| CH2CHCN | 10 | <0.05 × 1012 |
Notes. The best-fit kinetic temperature, Tk, used in the RADEX calculations for CH3OH is 7.0 K (also used for 13CH3OH), and for HCOOCH3 A it is 27 K. For all methanol species and CH3CHO a source size, θsrc, of 115″ was used. In the LTE method, we assumed a fixed Tex without an associated error. Here, ‘RD’ denotes for which molecules the rotation diagram method was used.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


